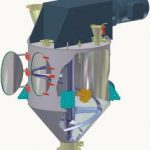
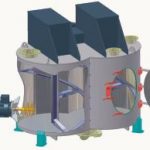
Although the system manufacturer has no influence over production-related QA systems or lacking instructions from pharmaceutical companies, he can make a significant contribution to system qualification, cleaning validation and risk analysis. For this reason, amixon GmbH has extended its range of services and now offers container mixers and reactors with appropriate certificates for mixing solid and semi-solid materials.
Stefan Ruberg
Cleaning validation of pharmaceutical mixers is an important part of the certification of the complete plant as legally laid down, for example, by the FDA. It has proved to be advantageous if manufacturers of process equipment take care of the testing and qualification procedures themselves and make the results available to the production facility for the purpose of validating the overall system.
Scope of the validation
The FDA definition of validation can be summarised as follows: Establishing documentary evidence to provide a high degree of assurance that a specific process will consistently produce a product in conformance with its pre-determined specifications and quality attributes. Validation can therefore be described as organised documentation of the production process. A distinction is made in detail between validation of the process, the plant and the computers. Process engineering and apparatus-related units have to be defined in order to qualify the production system. The key questions are similar to those for the HACCP concept:
- Where is there a potential hazard for the product?
- Which structural components may cause this hazard?
- How can the hazard be avoided?
When limited to the qualification of mixers and reactors, this means emphasising the design and functional properties and evaluating their potential risk to the prod-uct. In addition, potential sources of contamination have to be identified, for example seals, sampling valves, suction devices, delivery and loading units, inspection openings or installations which can be dismantled. The latter components are particularly significant if the production plant is used for different materials that are perishable or susceptible to contamination. They are, however, equally important if toxic intermediate products are generated as well as in the presence of highly concentrated active ingredients. Moreover, cleaning procedures and cleaning zones have to be defined as early as the design phase. It must be possible to decouple the plant at its interfaces and close it off. The cleaning process also includes discharge of the washing water, removal of the fumes and the drying check for the plant. Occasionally, it may also be necessary for the equipment to be steam-sterilised and cooled.
From design onwards
Qualification, therefore, begins at the design stage (DQ), in that the recommendations of the EHEDG and the regulations of the 3a Sanitary Standard have to be complied with by design and production engineers alike. The cleaning procedure is determined by the cleaning validation. According to Professor H. Reuter (University of Kiel), there are six so-called cleaning factors, the simultaneous combination of which is largely responsible for the success of the cleaning process. The process itself determines the type and quality of contamination and the system design. The four remaining factors, on the other hand, can be influenced to a certain extent:
- Chemical activity of the cleaning agent
- Duration of the cleaning process
- Temperature of the cleaning agent
- Inflow and outflow conditions of the cleaning agent (pressure, volume flow, angle of incidence, turbulence)
The type of contamination to be treated is important. If it is adhesive film from a liquid or loose powder deposits, the cleaning process is comparably simple. However, this is only the case if all surfaces of the apparatus – including the feed and discharge connections – are sprayed with cleaning fluid and the discharge outlet allows the dirt to flow away efficiently. On the other hand, residues which have become abraded or adhere, or which have dried, require much greater cleaning effort. The challenge is to optimally balance the above-mentioned cleaning factors. It is not possible to compensate for a failure to observe the basic principles of hygienic design, even with a high surface quality, i.e. low surface roughness. Tests have shown, for example, that a minimal surface roughness of Ra = 0.2 µm does not necessarily improve the cleaning result but in individual instances can actually make it worse. A mean roughness figure (Ra) of less than 0.8 µm has proved effective in practice.
Amixon mixers and reactors satisfy the strict requirements of the “EHEDG for dry and wet products” as well as the requirements of the “3a Sanitary Standard“. All important details are documented in conformance with FDA regulations. Amixon equipment combines the following quality features:
- All the bearings in the mixing device are located above the processing chamber, from where it is driven. The mixing tools have streamlined contours and smooth curves, with neither screw fittings nor flanges.
- The processing chamber is equally smooth: right-angle connections are implemented as throats with a radius >8 mm.
- The seals of the mixing device shafts are either sterile axial seals or gas aperture seals.
- The inspection openings have been kept free of inactive areas by using the “Clever-Cut-Design“ (www.clever-cut.de). The seal is provided here by an FDA-compliant polymer seal with no dead space, located close to the product.
- The mixer’s outlet connection has a gas-proof seal and no dead space. This fitting is provided with separate cleaning equipment.
- All parts that come into contact with the product are smoothly machined. The mean roughness value (Ra) is <0.8 µm.
- All items mounted on or in the equipment are installed with aseptic, quick-locking mechanisms. Orbital washing heads can be introduced to the mixing container in just a few manual steps.
- Water-carrying parts such as washing balls, rotary washers or high-pressure orbital nozzles are removed from the processing chamber once cleaning has been carried out. This is the only way to avoid the risk of residual contamination.
Requirements and evidence of hygiene
In practice, the mixer to be validated is first evaluated on the basis of its overall appearance and the design of all parts that come into contact with the product. The evaluation criteria are mentioned above. To begin with, there is a defined and reproducible level of contamination, such as will develop after a typical period of real production. A precisely specified cleaning programme then takes place. All influenceable cleaning parameters are strictly defined:
- Chemical activity of the cleaning agent
- Duration of the cleaning process
- Temperature of the cleaning agent
- Inflow and outflow conditions of the cleaning agent
To provide evidence of hygiene, the previously cleaned and dried mixer under test is contaminated with an easily detectable chemical that has a similar or identical behaviour to that of the real material. Cleaning and drying are followed by testing of all parts that into contact with the product. These parts are rubbed down with a small sponge on which the test chemical can later be detected. The test results serve as a starting point for improving the above cleaning factors.
Deliberate, prior contamination with spores and fungi is a variation of the swab method. The advantage of this cleaning test is that the success of the cleaning process is quantifiable and easily replicable. The disadvantage is the time necessary to grow the swab cultures in solutions. A bioluminescence detector is used specifically to furnish quantitative proof of micro-organisms, fruit cells and liquids. The energy-storing substances present in all living cells are revealed by a Luciferin-Luciferase reaction in the form of relative light units.
Since the process is quick, simple to use and also easily replicable, it is often preferred. It is important for the cleaning results to be transferable to as many sizes as possible in each series. Validation of a particular mixer/reactor can be carried out quickly on the basis of experience gathered from several hundred amixon mixers. In addition to validating cleaning, amixon can also, if desired, take care of the mixer qualification (DQ, IQ and OQ), perform individual tests and supply full documentation. Finally, the functional qualification is demonstrated in the presence of the production manager.
cpp 456
MischerExpo – Virtual Exhibition for mixers, agitators and kneaders
Technopharm 2005
More information on the mixers
Share: