At the AstraZeneca factory in Plankstadt, purified water is used, amongst other things, for the final rinse and for cleaning surfaces in contact with the product – a common practice in the pharmaceutical industry. However, the water is produced and used in a Class 1 hazardous area where extremely flammable solvents are also employed. Under these conditions, any reduction in quality or reliability is unacceptable.
Christian Stark
For many people suffering from schizophrenia, Seroquel – a medicament with the active ingredient quetiapine – offers a chance to lead a normal life without the risk of side-effects frequently encountered with other drugs, such as uncontrolled movements or increased weight. Due to its excellent properties, Seroquel has in the past few years become a blockbuster for AstraZeneca. The company, with its headquarters in London, is a world leader when it comes to research into therapies for diseases of the gastrointestinal tract, the cardiovascular system, the respiratory tract, oncology and the central nervous system. In 2004 alone, the budget for research and development was 3.8 billion US$.
The company has two facilities in Germany – in Wedel and Plankstadt – with a total of about 2400 employees. The actual synthesis of the active ingredient is carried out in England, while Plankstadt is responsible for the final step in the production of quetiapine. The substance is subjected here to a multi-stage crystallisation process with unusually high quality standards. After ten to twelve hours of crystallisation, it is centrifuged, dried and ground to the desired degree of fineness. It is then shipped to the UK and the USA for pressing into tablets, packaging and worldwide distribution.
Approximately 160 to 240 t of the substance are processed in Plankstadt every year. Although the plant was built in 1978, it certainly does not look its age. Since the merger of Astra and Zeneca in 1999, the company has invested more than 80 million US$ in the facility. In 1995/96, part of this amount was allocated to a new purified water system consisting of a water softener, a two-stage reverse-osmosis unit, a UV disinfection stage and a hot-storage tank conforming to USP 23. With the introduction of USP 24 a few years later, the company decided to add an electro-deionisation unit and an online TOC to the system, since at the time one substance was still being processed with purified water. “We are now very glad we made the decision back then,“ says Elmar Wenzel, Plant Manager at AstraZeneca, “although we did not actually need the higher water quality immediately. However, in the face of the global transformations in the pharmaceuticals market, we need to be able to react quickly to product changes.“
Trouble-free commissioning
The decision to use the Septron EDI technology from Christ was an easy one, since the pharmaceutical production facility already had a water treatment system from the same supplier. This facility manufactures products such as Niften, Inderal, Ternomin and Casodex. About three months after the initial planning phase, the Septron unit was put into service with a capacity of 2,500 l/h. After a further three months, the system was validated without problems thanks to the package offered by Christ, which included installation and operation qualification. The Christ concepts for design, installation, operation and performance qualification relieves users of the need to carry out time-consuming approval procedures.
Multiple safety
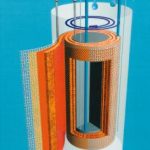
The permeate produced by the existing two-stage reverse-osmosis unit is fed to the Septron 2.500 PH. The RO unit removes about 98 to 99 % of the minerals from the water, while the electro-deionisation unit with DC field extracts the remainder. The patented electro-deionisation module operates with a water output of almost 100 % and needs no chemicals whatsoever. Each module consists of two chambers, separated from each other by special membranes. The purified water chamber is filled with a special ion exchanger resin. As the permeate flows through this chamber, almost all of the ions still contained in it are removed. The resulting diluate is distributed to the consumers. A DC voltage applied across the module splits some of the water molecules into hydrogen (H+) and hydroxide (OH-) ions, which continuously regenerate the ion exchanger resin in the module. In the concentrate chamber, the ions removed from the permeate are transferred to softened rinsing water running through it, which is then discarded to a drain.
The very high pH gradient in the Septron module – between 2 and 12 – restricts the growth of germs to a minimum during operation. The TOC figure is also considerably lower. Furthermore, the electro-deionisation module reduces the number of pyrogens in the water. The limit values for conductivity are, accordingly, well below the required value of 1.3 mS/cm. The system design with no dead spaces, the automatic disinfection process and the use of materials such as stainless steel and PVDF protect the water against secondary contamination. The module also helps to save energy and costs, since it operates with only a low DC voltage.
If the system is operated intermittently, there are idle periods with a risk of contamination. For this reason, the system automatically switches to circulation mode when there is no demand from the storage tank. If the water temperature rises above a certain value during circulation, it is automatically discarded. As soon as water is needed to top up the storage tank again, the system returns to its normal operating mode and purified water enters. When the tank is full, the diluate is returned to the distributor.
Special explosion protection
The electro-deionisation unit at AstraZeneca is the only one in Germany which is located in a Class 1 hazardous area. ”Our entire production facility is designed with explosion protection,“ explains Christoph Probst, the leading qualification engineer at AstraZeneca. “After all, about 20 t of highly flammable solvents flow through our production system every day – and the possibility of a leak can never be completely ruled out. Since many patients depend on the medicament, to interrupt our production process due to a fire or a similar problem would have serious consequences.“ The subject of safety therefore has top priority. “A lack of space prevented us from installing the water treatment system elsewhere,“ says Wenzel. “One option would have been a separate room, but this would have involved expensive reconstruction work.“
Whereas explosion-protected versions of components such as instruments, motors and pumps are available as standard, this was not the case with the Septron and the UV disinfection unit. It was therefore necessary to encapsulate these two components. The required protection was achieved by installing the complete electro-deionisation unit in a pressurised housing (E Ex p), which is filled with inert gas at a pressure above atmospheric in order to prevent the ingress of explosive gas mixtures. In “leakage compensation“ mode, the unit is rinsed and a pressure of at least 0.8 mbar above atmospheric is maintained in the E Ex p housing. If the external compressed air supply fails, a special interface relay with explosion protection disconnects all wires which are not intrinsically safe. Both units were individually inspected and approved by the responsible inspection authority. “In the end, the explosion-protected version was easier to build than we expected,“ says Probst.
Tested safety
“Our water is always tested critically, although no purified water is needed for 80 % of the processing steps for active ingredients because, for example, crystallisation takes place in ethanol,“ says Wenzel, fully aware of the sensitive nature of the subject. For this reason, water tests are part of the daily routine in Plankstadt. These tests include the extraction of water samples and online monitoring of TOC values, conductivity and temperature. If, for example, the conductivity exceeds the preset limit value, “Conductivity bad“ is indicated on the control cabinet and the system is rinsed and shut down. The permeate, rinsing water and diluate pressures are monitored by special switches. If a pres-sure rises above its defined limit value, an alarm is generated and the system likewise shuts down. The same happens if the compressed air or electric power supply fails. In the latter case, the system stops and all valves are set to their safety positions. When the power is restored, the system is started up automatically as defined by the control program. The system executes a self-diagnosis and then switches to “standby“ or “water production“ mode as applicable. If the compressed air supply fails, all valves are set to their safety positions and the system is shut down. This triggers a rinsing operation in which the concentrate side of each Septron module is first rinsed with permeate before switching off the modules. All data is transferred to a process controller. “This ensures that each batch is fully documented in a production record file,“ says Probst.
cpp 481
Septron and other Christ technologies
Product database
Share: