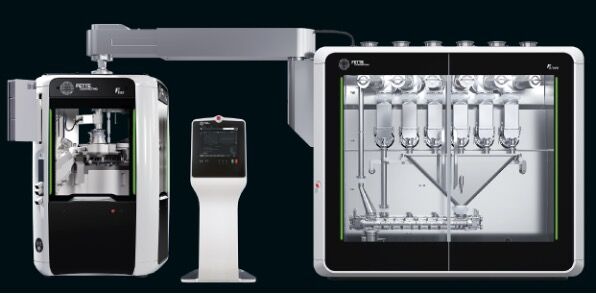
Instead of separate production steps, continuous manufacturing (CM) allows ongoing processing from the powder feed-in to the finished tablet. It offers an accelerated adaptation to the changing requirements in oral solid dosage (OSD) production. Nowadays, the system design can be implemented in a space-saving manner, with simple installation and intuitive operation. Thanks to integrated, controllable processes, CM is generally also more reliable and efficient than many batch-to-batch processes
For tablet production, Fette Compacting has rethought the continuous direct compression process from scratch. The powder is fed from the FE CPS continuous processing system into the tablet press without additional granulation. Compared to granulation-based production, several steps are eliminated, which reduces space requirements and energy consumption and makes the process leaner overall.
The system is capable of processing a wide range of ingredients in a variable throughput range of 5 to 200 kg/h. It can dose and mix up to six powdered raw materials and transfer them to the downstream process. The system unit, together with the tablet press and terminal, can be installed on a single level, facilitating its integration into existing production environments.
For the first time, Fette Compacting has also fully integrated the associated sensors for quality control into the system and its control unit.
Fette Compacting
Achema: Hall 3.0, Stand F3