Cost is a critical factor in the large-scale production of fine chemicals and the demand for efficient synthesis routes is correspondingly high. The optimisation of chemical reactions and industrial processes using catalysis as a key technology enables a more sustainable use of raw materials and energy sources as well as loss-free utilisation of the raw materials. A tailor-made catalyst accelerates the reaction, prevents the formation of by-products, and thus reduces the energy requirements of the entire process. Carbonylation reactions are among the most important applications of industrial catalysis. The catalysed introduction of the CO group takes place through the use of carbon monoxide (CO), which, as an important C1 building block of the chemical industry, can be extracted both from fossil resources (coal and gas) and from renewable sources (CO2 or biowaste). Carbonyl compounds (aldehydes, acids, esters) are produced annually on a scale of several million tonnes and are used for numerous consumer goods.
Direct carbonylation of 1,3-diene
Despite the discovery of homogeneously catalysed carbonylation processes almost eighty years ago, challenges remain unsolved, especially the direct dicarbonylation of 1,3-dienes. This reaction is a so-called dream reaction and enables a more environmentally friendly, atomically efficient production of adipate diesters, which are produced on a large scale as building blocks for polyamides and polyesters. In particular, adipate diesters are used in plasticizers, perfumes, lubricants, solvents and active pharmaceutical ingredients. A further application is the production of nylon. Currently, adipic acid diesters are produced industrially by oxidising a mixture of cyclohexanol and cyclohexanone with an excess of nitric acid. The resulting adipic acid is esterified with the corresponding alcohols. This process requires special equipment due to the corrosive effect of the acid. It also releases nitrous oxide (laughing gas), which on the one hand binds stratospheric ozone and on the other hand is 300 times stronger as a greenhouse gas than CO2.
Therefore, the work of the scientists at the Leibniz Institute for Catalysis (Likat) –
Prof. Dr. Matthias Beller, Dr. Ralf Jackstell, Dr. Helfried Neumann, Jiawang Liu, JiYang – in collaboration with Prof. Dr. Robert Franke, Evonik Performance Materials GmbH and Associate Professor of Chemistry at the Ruhr University Bochum, has great potential for carbonylation chemistry. They developed a palladium catalyst with a specially designed pyridyl-functionalised bisphosphine ligand that enables the highly selective and efficient double alkoxy carbonylation of 1,3-butadiene into adipic acid esters in a single step. The key to success was the ligand design. The combination of a bidentate phosphine ligand with a basic pyridyl substituent on the phosphorus and a palladium precursor delivers a yield of ≥95 % and selectivity of ≥97 % in the dicarbonylation of 1,3-butadiene dialkyl adipates.
The right catalyst is key
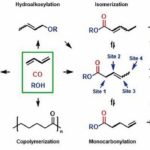
The difficulty lies in the complexity of the dicarbonylation reaction, which usually tends to form numerous by-products. The simultaneous introduction of two CO groups presents several challenges in this catalytic process:
- The simultaneous production of two different carbonylation reactions on a diene substrate (no other research group has been successful before)
- The selective formation of the linear dicarbonylation product, although the isomerisation of the originally formed monocarbonylated intermediate to the terminal olefin is thermodynamically particularly unfavorable
- The suppression of other side reactions such as telomerisation, hydroalkoxylation and (co)polymeridation
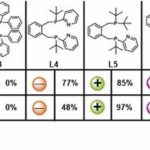
For the development of a suitable catalyst, palladium-coordinated, base-modified derivatives of the 1,2-bis[(di-tert-butylphosphino) methyl] benzene ligand (L1, dtbpx), which is used for the large-scale production of methyl methacrylate, were chosen. First optimisation studies with this ligand showed low activity but good selectivity. The incorporation of suitable basic groups leads to a significant increase in activity. However, as the direct comparison between L1 and the doubly base-modified L4 shows, this is at the expense of selectivity. The solu-tion was to combine both structural ele-ments. The specially developed L5 (HeMaRaPhos) combines the excellent selectivity of L1 with the high reactivity of L4.
Supplementary studies on the performance and optimisation of the catalyst system now allow the direct dual carbonylation of 1,3-butadiene to adipate diesters with high catalyst conversion rates (60 000), yields of up to 95 % and an outstanding selectivity of over 97 %. The catalyst system can also be transferred to other dienes, paving the way for a completely new synthesis route for the production of many fine chemicals.
Evonik Performance Materials GmbH, Essen