Most of today’s chemical and pharmaceutical production runs on special process equipment. With the need to process increasingly aggressive and potent compounds, chemical engineers and equipment suppliers can now add seal customisation to their armoury. The ability to fine-tune the physical properties and chemical resistance of the seal can reduce seal maintenance, allow greater operational flexibility and ultimately increase plant productivity.
David Holt
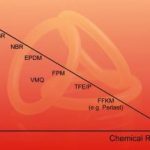
Selecting a seal begins with meeting the requirements for chemical resistance and operating temperature of a process. It needs to be borne in mind that synthetic elastomer materials generally consist of an organic polymer and inorganic reinforcing filler systems. Although the polymer backbone may be similar, thus determining many of the physical properties (Fig. 1), there can be significant differences in the cross linking and filler systems, creating many of the differences in physical properties and hence sealing efficiency.
Seal customisation is possible for the physical properties of an elastomer. By making changes to the filler system, it is possible to optimise the physical properties of a particular grade of material when compared to others within the same grade. The reinforcement effect of a filler is complex and dependent upon its structure, particle size and the chemical make-up of the particles themselves. Carbon black, for instance, has a very irregular surface, which makes the reinforcement particularly effective. However, some synthetic silicas are perfectly spherical, offering very little in terms of reinforcement. In order to achieve specific physical properties from a material, the correct combination of reinforcing and non-reinforcing fillers must be selected.
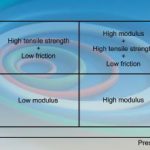
Fillers can be classed as reinforcing or non-reinforcing, depending upon whether they arrest crack propagation to a greater extent than they raise stresses or vice versa. Within a polymer the filler can have two effects: it may act as a stress raiser, reducing the energy at break, or it may arrest crack propagation to increase the energy required for breakage. A simple summary of the physical properties that may be optimised through the use of fillers is shown by Figure 2.
Engineers can use the filler effect to select the quadrant of the Physical Properties Box best suited to their process equipment. The modulus of a material is related to its hardness. As the modulus increases, so does the hardness; O-rings of high hardness are more capable of withstanding extrusion to higher pressures. The ultimate tensile strength, elongation to break and hysteresis loss of a material are useful indicators of the elasticity of an elastomer. Although seals by their very nature are typically used in compression, their elastic properties can result in the development of tensile stresses within the body of the seal when subjected to compression or shear stress.
The right decision
Typical dynamic applications include sealing against a reciprocating or rotating shaft or bore. The compression of the material, combined with shear frictional forces, can result in tensile stresses that exceed the ultimate tensile strength of the material, causing a tensile failure. In this case using a large surface area, small particle size filler would be a better choice than the standard reinforcing filler. However processability may suffer and scorch may result. It is often the case that a compromise between property enhancement and processability has to be made. Clearly, the selection of filler systems must be such that it achieves the required customisation of physical properties without adversely affecting the chemistry of either the polymer cure, or the elastomer’s final chemical resistance. For these reasons it should be remembered that fillers introduce chemical effects. Even though solid, the filler will have an associated acidity or alkalinity (pH) which, in rubber chemistry, is very important; even slight changes in pH can greatly affect the curing process. In carbon black (dependent on the specific grade) the particles can be slightly acidic, which can lead to a slight retardation of the cure. For some silicas though, the pH can be as great as 4.0, which is very acidic – if these are not compounded properly, the cure can be completely ineffective.
Further complications are also found with filler selection where the chemical compatibility can be greatly affected. Each filler has an associated pH – this is essentially a function of the molecular groups on the surface of the particle and the media into which it is immersed. Using silica as an example, which has a fairly acidic pH, in alkaline media, the silica will try to absorb the alkaline in an attempt to reach neutrality, causing significant swelling of the seal.
Design with nano-fillers
A new development in sealing is the introduction of nano-fillers. Nano-scale filler systems greatly increase the surface area-to-volume ratio of the filler system and as such can significantly increase the extent to which a single physical property, or group of properties can be modified. As with traditional fillers, different nano-fillers can be used to achieve different physical properties and the selection of fillers remains just as critical.
Low-pressure, chemically aggressive processes require different physical properties that are less obvious in nature. Low-pres-sure applications, such as vacuum applications, rely upon the initial compression of the seal rather than the differential pressure to generate the normal sealing forces. These normal forces are far lower than those generated in high-pressure applications, meaning that high-modulus, hard materials are unable to conform to the mating surfaces during sealing. Hence, low-modulus materials can be used for low-contact-force applications.
Lower modulus equates to lower stresses within the elastomer for a given strain. Reducing the stress levels in an elastomer material will reduce the likelihood of chemical attack. Stress induced chemical attack is a macroscopic material failure due to mechanically propagated cracks, initiated by chemical degradation of the material.
Stress induced attack
To understand stress induced attack fully, it is necessary to appreciate the nature of the material. An elastomer is made up of long chains of molecules onto which are bonded other atoms or groups of atoms. Locations within the cross linking molecules or unsaturation on the backbone typically contain the weakest bonds. Those are locations which are the first to be chemically attacked. By applying a stress to an elastomer, we deform the molecular chains, which both reduces the energy needed to break covalent bonds and introduces steric twisting that exposes weaker bonds; this mechanism is known as “stress induced chemical attack”. The chemical attack initiates the formation of microscopic fissures. Once formed, a crack will continue to propagate, growing slowly until it reaches a critical length, at which point the fracture energy causes rapid propagation of the crack across the whole section of the seal, causing the seal to break in a brittle-like manner.
When operating in chemically aggressive, low-pressure environments, we must therefore look to introduce filler systems that enhance tensile strength and elongation at break, but offer the low modulus needed to achieve good compliance and prevent accelerated chemical attack.
Spherical perfluoropolymer filler system
Nano-filler technology has been used to optimise perfluoroelastomer characteristics for low-pressure, chemically aggressive, environments such as those seen in bio-analytical equipment. It utilises a spherical perfluoropolymer filler system of 30 to 35 nm diameter that enhances both tensile strength and elongation at break, yet maintains a low modulus. The perfluoropolymer fillers are highly inert, preventing chemical attack of the fillers. By offering a low modulus of 3.5 MPa, the perfluoroelastomer offers high sealing efficiency at low pressures and minimises stresses within the polymer. By minimising stresses, the perfluoroelastomer offers far higher resis-tance to stress induced chemical attack than conventional perfluoroelastomers, proving time and again that it maintains excellent chemical resistance under actual process conditions.
cpp 405
More information about seals from PPE
What is Perlast?
Share: