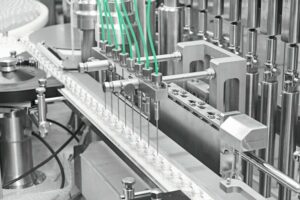
Anyone involved in pharmaceutical production who wants to exploit the opportunities for growth associated with new, highly active substances, has to have safe and efficient containment solutions.
But that’s not all. Protective measures are increasingly also required for a large number of medicaments that in the past have been manufactured without taking special precautions. Low-dust production systems are the new minimum standard.
In practice, however, precise specifications for the individual process steps have not been available until now – in spite of comprehensive guidelines and stricter enforcement by regulators.
Fette Compacting is therefore taking the first step in the area of tablet production. The Containment Guard is the first quality certificate to determine and document the retention performance of containment tablet systems even before the operator’s final risk assessment. It is both a test procedure and the foundation for the technical development of containment solutions in tablet production. The Containment Guard additionally offers comprehensive service, training and consultation, precisely matched to the needs of production under containment conditions.
Based on toxicological limits
The toxicological assessment of the active ingredients to be processed by the manufacturer of the medication forms the basis for the containment system design. Manufacturers determine the maximum permitted limits from this assessment. This process involves the use of models such as the occupational exposure limit (OEL), the permitted or acceptable daily exposure (PDE, ADE) or the maximum workplace concentration (MAK). The limits specified in this way serve as a reference for the subsequent risk assessment when the overall system is put into operation based on the standardised measurement of equipment particulate airborne concentration (SMEPAC). This provides a suitable framework for general assessments of a system’s containment quality.
To enable a reproducible assessment specifically for containment tablet systems, the Containment Guard supplements the SMEPAC guideline with further, practically relevant aspects:
- Positioning of the measuring probes
- Whereabouts of the operators
- Number of samples taken
- Operating states of the machine
- Behaviour of the system in the presence of faults or malfunctions
- Calculation of the system’s overall performance, including process equipment
Precise measurement, low expense
The measuring criteria used by the Containment Guard correspond to those of the SMEPAC guideline. However, this method always covers the complete system, including the process and safety equipment.
The structure and sequence of the test procedure are likewise standardised. Fette Compacting performs the test in a specially equipped room at its Customer Centre in Schwarzenbek. After passing the test, the equipment receives the Containment Guard certificate. Fette Compacting hands over the documentation together with the system. This forms the basis for the operator’s risk assessment and reduces ongoing expenditure after the plant has been put into operation.
Multi-stage model
As a complete solution, the Containment Guard takes in all tabletting system components. This particularly refers to third-party process and safety equipment as well as Fette Compacting’s patented air management system. The individual stages of the Containment Guard are oriented towards the pharmacological and toxicological classification of the active ingredient (occupational exposure band, OEB). The OEB levels are clearly illustrated in the containment pyramid of the International Society for Pharmaceutical Engineering (ISPE). Containment solutions for tableting at OEB Level 3 or higher – i. e. substances with a low pharmacological effect – are relevant in practice.
Level 3: Low-dust standard
In the light of the increase in potentially hazardous substances, it can be assumed that products incorporating substances with low pharmacological activity and with relatively low dust exposure will become the minimum standard for tablet production. Efficiency is the central key to lower manufacturing costs. Fette Compacting’s FE55 and FE75 tablet presses are the ideal technological solution here. When fitted with an optional containment package and associated process equipment, they form the basis for Containment Guard 3 upward.
In the FE series, the tabletting process is fully automatic. From the filling of the machine to tablet discharge, containment is unbroken. The machines are accessible from every side through glove ports. The containment package for the FE55 and FE75 includes a flap valve for secure product feeding, hermetically sealed and automatically lockable window flaps, rapid transfer ports (RTP), a low-dust tablet outlet, H13 HEPA filters, a hand vacuum hose for initial manual cleaning, an interface for the process and safety equipment and a special software safety concept utilising the human-machine interface (HMI).
Level 4: Optimised cleaning
When processing highly active and toxic pharmaceutical ingredients, the use of wash-in-place (WiP) systems significantly reduces the exposure of machine operators during cleaning. The effort required for product changes is also reduced, so that there is far less machine downtime. The containment tablet presses in Fette Compacting’s i-series are supplied with a manually operated washing gun and a vacuum cleaner nozzle that easily remove product residues from the compression chamber. An RTP access is likewise integrated for inserting and exchanging tools.
Level 5: Maximum safety
Encapsulating tabletting solutions that meet Containment Guard 5 offer maximum safety in production as well as optimum protection for operators. They are based on Fette Compacting’s isolator technology. The isolators can be fitted with a range of options such as an upward de-duster, a metal detector and an IPC Checkmaster for in-process monitoring. All transfer interfaces and flaps of the plant are sealed absolutely tightly. The operator can carry out all the work required on the machine or in the isolator with the help of the integrated glove ports and RTP accesses. Individual compression and in-process monitoring steps can be supervised via the HMI. The same applies to air cleaning, which the operator can monitor using the software-controlled air management system, including a vacuum emergency system. In combination with a WiP Centre the plant can be cleaned automatically in a very short time.
Online search: cpp0317fette